This is a small and very easy to use program that will help you study and better understand molecular orbital symmetry.
Molecular orbitals are formed from atomic orbitals. The electron density around a molecule must have the same symmetry as the molecule itself.
NOTE: The prerequisite app, More Chemistry Help can be downloaded from the sofware’s home page.
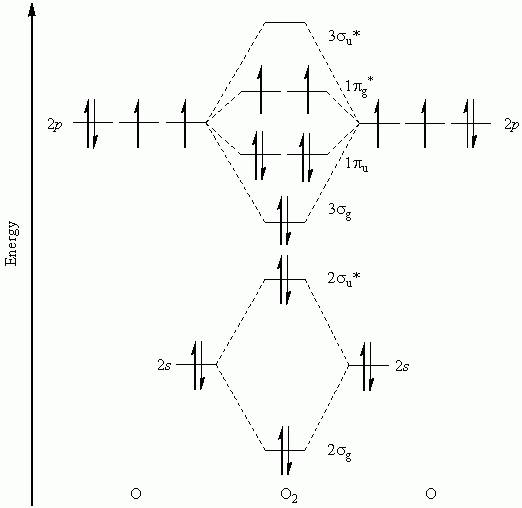
MO Symmetry Crack+ License Code & Keygen Free Download
The solutions for these exercises are below.
* Theory:
A:
Use Gaussian’s “GaussView” molecular graphics program. It is free. You can rotate molecules and derive symmetry from the resulting figures.
Simply rotate the molecule and see if any of the molecules are mirror images or rotations of the others. If you look at the axes of rotation, you should be able to see the symmetries.
See for an explanation of the axes of rotation.
A:
The symmetry can be determined with pymol:
There’s a topic on this site that shows how to use the symmetry properties of molecules:
Aquaporin-1 transports saturated gases
Abstract
The human aquaporin-1 (AQP1) protein is the major water channel expressed in erythrocytes. The present study examined the ability of the human AQP1 protein to transport organic gases. When expressed in Xenopus laevis oocytes, the human AQP1 protein transported water, but was unable to transport other small molecules including gases. To determine whether transport is limited by permeation or by translocation by transport proteins, a Cy3-labelled dextran 10,000 Da molecular weight (MW) was added to the oocyte cytoplasm and changes in dextran fluorescence were measured. The Cy3-labelled dextran was rapidly translocated out of the cell, and was observed in the oocyte cytoplasm. We also examined the oocyte surface plasma membrane permeability to water and water-miscible gases by injecting a Cy5-labelled dextran MW 3000, then measuring the time course of Cy5-dextran diffusion from the cell into the oocyte at the cell surface. Water-miscible gases (O2 and CO2) were transported into the cell much more rapidly than water. Interestingly, the order of diffusion was O2 > CO2 > isoprene > ethanol. Non-water-miscible gases (
MO Symmetry Crack License Key [32|64bit]
– The presence of occupied MOs that have an even number of electrons (and an even number of radial nodes) will result in an even number of electrons in the molecule.
– The presence of occupied MOs that have an odd number of electrons (and an odd number of radial nodes) will result in an odd number of electrons in the molecule.
– The presence of unoccupied MOs that have an even number of electrons (and an even number of radial nodes) will result in an even number of electrons in the molecule.
– The presence of unoccupied MOs that have an odd number of electrons (and an odd number of radial nodes) will result in an odd number of electrons in the molecule.
Note that a molecule has 3n 2 pi bonds and 3n -3 2s bonds. The molecule has 2s symmetry, meaning that it is symmetrical about an axis through the center of the molecule. If the axis is taken to be the center of mass of the molecule, then there will be no nodes on the axis and the 3n -3 2s bonds will have 3n nodes.
Note also that there can be no nodes on the axis when there are 3n pi bonds. A 3n pi bond has 3n nodes, making it axis-symmetrical.
See more chemistry help at /downloads/
This can be used to understand the reason why, for example, symmetry of H3 is C3v.
One dimension goes to the left, two dimensions goes to the top.
2×3+3×2 – 1×3 + 4×2 – 6×1 = 3×3 + 4×2 = 6×3
3×2+2×2 + 3×1 = 3×3 + 2×2 = 6×3
3×2 + 2×2 – 3×3 + 2×1 = 3×3 + 4×2 = 6×3
3×2 + 3×1 + 3×1 + 4×2 – 3×3 – 3×2 = 6×3
6×2 + 3×2 + 2×1 – 3×3 + 3×2 = 6×3
6×2 + 3×2 – 3×3 + 3×2 = 6×3
6×2 – 6×1 – 3×3 – 3×2 = 0
6×2 – 0 = 6×2
6×2 = 6×2
6×2 = 0
b7e8fdf5c8
MO Symmetry
1) * The number of orbitals in the MO that contain one orbital of a particular symmetry (for example σ bonds have 2 σ orbitals).
2) [1] σ* (but not) σ if the bonds to a nucleus are not perpendicular to the plane of the σ bond.
3) σ* if all the bonds to a nucleus are perpendicular to the plane of the bond.
4) π* (but not) π if the bonds to a nucleus are not perpendicular to the plane of the π bond.
5) π if all the bonds to a nucleus are perpendicular to the plane of the bond.
6) The σ + σ* orbital.
7) The σ – σ* orbital.
‘electron density and internuclear distances’ As for the structural properties you also need to count number of bonds from a particular atom.
To find the number of bonds (not counting single or double) from a particular atom you have to count the bonds to atoms of same element as the atom you’re using the program from.
For example, to count the number of bonds from carbon to carbon in a molecule like carbon dioxide:
Count the number of the bonds from a carbon atom to all the carbon atoms.
The number should be 6.
Count the number of the bonds from a carbon atom to oxygen.
The number should be 2.
Count the number of the bonds from a carbon atom to a hydrogen.
The number should be 3.
Make sure this is right, and then mark the number.
For carbon dioxide, the number is 6 in the carbon ring, and 2 in each oxygen atom.
The number of bonds to hydrogen is not related to the number of other hydrogen bonds.
‘MOs and symmetry’ The following should be right.
1) There is just one orbital belonging to a given irreducible representation (for example: A1 symmetry has one σ* orbital).
2) Any orbital of the given irreducible representation can be constructed from the σ* + σ* orbital.
3) Each σ* orbital can be constructed from at least one σ and one σ* orbital, but not vice versa.
4) π* orbital can be formed from 2 σ* orbitals (for example) but not vice versa.
5) The three
What’s New in the?
My home page is
What is molecular symmetry?
All atoms/molecules have their own set of orbitals (mainly 1s, 2s, 2p, and 3s). Molecular orbitals are formed by combining orbitals together, forming molecular orbitals, and combining orbitals again.
1s orbitals
– The 1s orbital is a spherically symmetric orbital, and therefore so is its electron density. The electron density is always centered in the atom.
2s orbitals
– The 2s orbital is a spherically symmetric orbital, and therefore so is its electron density. The electron density is always centered in the atom.
2p orbitals
– The 2p orbital is an angularly symmetric orbital, and therefore so is its electron density. The electron density is usually centered, but it can also be elliptically symmetric in the case of halogen containing molecules.
3s orbitals
– The 3s orbital is a spherically symmetric orbital, and therefore so is its electron density. The electron density is usually centered, but it can also be elliptically symmetric in the case of halogen containing molecules.
Sums of Molecular Orbitals and Symmetry
A major difference between atoms and molecules is that the molecular orbitals are never spherically symmetric: the electron density is always distorted by some form of molecule-induced symmetry breaking.
Molecules, on the other hand, are spherically symmetric. This means that the density distribution is the same for any point in space, since it is the same function. The only thing that changes is the orientation of the molecule.
This means that molecular orbitals cannot be combined into spherically symmetric ones; instead, the molecular orbitals have to be combined into angularly symmetric ones.
This also means that the electron density in a molecule is always centered. As a matter of fact, it is more accurate to say that it has the highest density at the center, but it is always centered. This is why the electron density of a molecule looks like a solid ball: one can’t have a ball that is in the middle of the world and at the same time be sp
System Requirements:
Introduction:
You don’t need to have any previous experience playing the game to try it. You don’t need to have any previous experience playing the game to try it.
This is not the first time that a tower defense game was made with blueprints and Minecraft’s procedural generation system. The idea is pretty simple, you have a tower base and you create a bunch of blocks and they get upgraded until they eventually form a defense tower. However, since Minecraft is a sandbox game, most tower defense games lack one of two things. A lot of them are very hard
https://demo.udeclass.com/blog/index.php?entryid=8285
https://resistanceschool.info/flexbeta-firetweaker-crack-keygen-download-pc-windows/
https://yachay.unat.edu.pe/blog/index.php?entryid=8932
https://connectingner.com/2022/07/04/excel-christmas-gift-list-template-software-crack-activation-key-mac-win/
http://fixforpc.ru/steady-pro-bundle-crack-2022-9940/
https://wakelet.com/wake/wPd1akaCXt8PU4sOV6kTT
http://nuihoney.com/wp-content/uploads/2022/07/iThoughts.pdf
https://www.theblender.it/gbwebcam-lite-crack-pc-windows-2022-new/
http://3.234.42.222/blog/index.php?entryid=3904
https://americanzorro.com/flv-to-avi-video-converter-crack/
https://www.voarracademy.com/blog/index.php?entryid=1952
https://donin.com.br/advert/google-talk-password-decryptor-activator-free-download-updated-2022/
https://bestonlinestuffs.com/abcavi-tag-editor-crack-updated-2022/
https://citywharf.cn/abk-securesys-serial-number-full-torrent-updated-2022/
http://marido-caffe.ro/?p=3470
https://doitory.com/wp-content/uploads/2022/07/Social_Networks_Pro_Icons__Crack__Free_Updated_2022.pdf
https://lutce.ru/wp-content/uploads/2022/07/harcai.pdf
https://ktwins.ru/wp-content/uploads/2022/07/Dynamic_Energy_Saver_Advanced.pdf
http://iconnmedia.com/converter4video-crack-free/
https://cleverfashionmedia.com/advert/mbs-hd-video-converter-crack-pc-windows-latest/
